

So, we will just consider one of the three to be the central atom and place the other two on lateral sides. Just like triiodide ion where all the atoms are iodine, here, all the atoms are oxygen. Thus, the total number of valence electrons in ozone= 3*6 Oxygen belongs to group VI of the periodic table with an atomic no of 8. The below discussion, therefore, will be based on finding out the Lewis Structure of O3. Here, we will be dealing with ozone, the molecular formula is O3. We need to check whether all the atoms inside the given molecule are maintained at their least formal charge. Here, we will focus on calculating the formal charge.
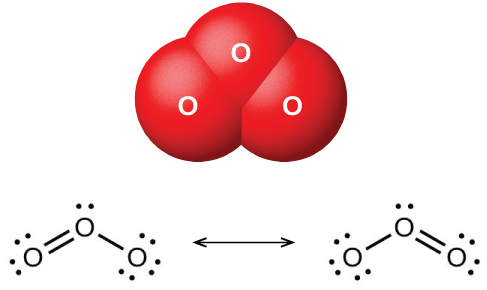
The bond formation (single and multiple ones) leads us to the final step of the process, i.e. We are now done with all kinds of bond formation, The last step is focused on the formal charge concept. Accordingly, multiple bond formation can be done. Once, octet fulfillment has been done, we now need to find out if bond formation is left. Starting with the electropositive ones, slowly fulfill the octet of the atoms. The fourth step of Lewis Structure formation is based on achieving this. So, the octet rule is based on the fact that every atom should have eight electrons in its valence shell. Hence, they react accordingly and tend to form more stable molecular compounds. This is carried out by sketching the skeleton diagram of the respective molecules as per requirementsĭo you know that when atoms contain less than eight electrons in their outermost electron shell, they are still in their reactive state? In this step, the task is to visualize the position of single bonds present in the molecule as a whole to the central atom. This atom will consist of higher sites of bonding compared to the others. The one with the highest valence usually has the least electronegativity. You can check this out by calculating the valence number. How can we do so? We can easily find the solution to this with one simple trick!įirst, point out the least electronegative atom. We now need to determine the central atom. While calculating the valence electrons, we need to work with these two signs. ‘-’ stands for the gain of electrons, or in other words, negative charge. ‘+’ stands for positive charge i.e giving away(loss) of electrons. The initial step towards forming this structure is to find out the total number of valence electrons. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding.Ī very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure: To be very precise, Lewis Structure is the name given to the structural representation of a molecule.


 0 kommentar(er)
0 kommentar(er)
